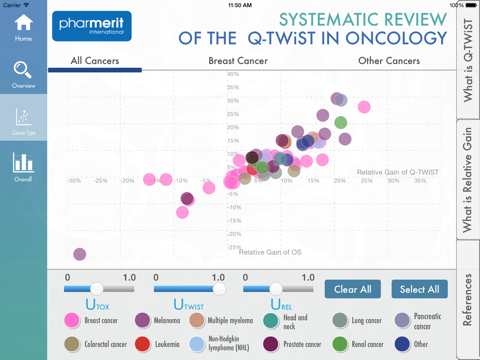
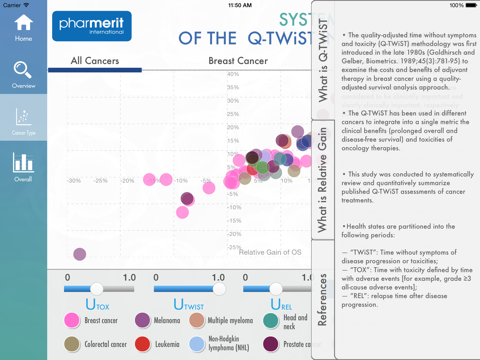
The quality-adjusted time without symptoms and toxicity (Q-TWiST) methodology has been used to assess the clinical benefits (prolonged [progression-free] survival) and costs (toxicities) of oncology therapies. This app summarizes a 45 articles published on Q-TWiST assessments of cancer treatments with a total of 69 Q-TWiST comparisons across 10 cancers, of which breast cancer was predominant.
Reported Q-TWiST information—includes time with toxicity (TOX), time before disease progression without toxicity (TWiST), and time in relapse after disease progression (REL)—overall, for breast cancer, and for all other (non-breast) cancers. Utilities for Q-TWiST were also captured, but within the app can be dynamically changed. The most commonly used utilities for Q-TWiST calculation were u(TWiST)=1, u(REL)=0.5, u(TOX)=0.5 (n=28, 62.2% of articles). The relative gain in Q-TWiST for active treatment arms was calculated as the difference in Q-TWiST divided by mean overall survival of control arm and plotted against overall survival. 41.8% of studies had a clinically important relative gain (≥10% ) and 17.9% had a clearly clinically important relative gain (≥15%) using their published utility values. The review of Q-TWiST for cancer therapies that is summarized in this app can serve as benchmark against which future analyses can be compared.